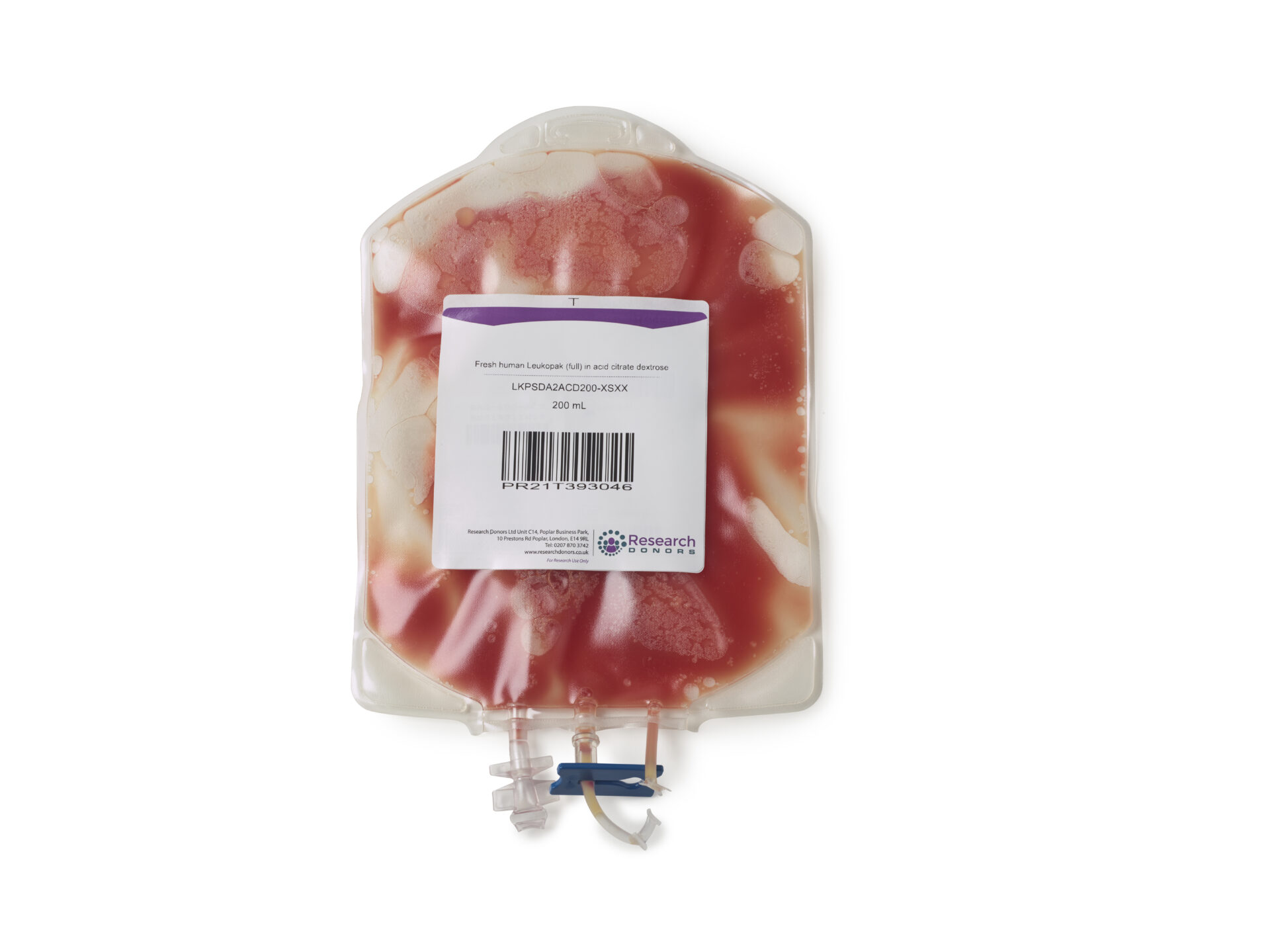
What is a leukopak?
Leukopaks are a bag of white blood cells collected via a process called leukapheresis.
Whole blood is passed through an apheresis collection system which effectively separates the blood fractions in a continuous flow process, based on density and then selectively removes the white blood cell component. The remaining blood volume is returned to the donor and the process continues for several hours during which time the entire blood volume of the donor may pass through the system 2-3 times
The resulting leukopaks contain a minimum of ten billion mononuclear cells (MNC’s) which are a group of white blood cells with a single nucleus.
Should I consider switching to leukopaks in my life science research project?
Leukopaks are essential in the development of cell therapies, serving as a rich source of peripheral blood mononuclear cells (PBMCs), such as T cells and natural killer (NK) cells. These cells are crucial for manufacturing advanced therapies, including CAR-T and TCR therapies, used in cancer treatment and other diseases.
In cell therapy manufacturing, the quality and consistency of leukopaks directly impact the success of the therapy. They provide starting material for genetic modification, expansion, and differentiation into therapeutic products. Standardized leukopaks from healthy donors allow for reproducible and scalable production, a critical step toward commercializing cell-based therapies.
Leukopaks also offer researchers in other areas of drug discovery, such as immunogenicity testing, the benefit of a huge number of cells from the same donor, collected at the same timepoint, allowing scalability and reproducibility not possible with other formats such as buffy coat.
Do you want to avoid donor to donor variability and the potential issues it can cause? Does your project involve repeated experiments over time or simply the need for large numbers of cells? If so, then leukopaks could well be the best option for you. Many of our customers have recognised these benefits and have now standardised on leukopaks for drug screening and safety assessment projects.
Why can I trust Research Donors’ leukopaks?
Our leukopaks are collected by experienced nurses who have been trained in leukapheresis by Terumo BCT, the manufacturer of the Spectra Optia® instruments we use for apheresis collections.
Our apheresis donors are carefully selected from within our large pool of whole blood donors, based on their suitability for apheresis collections, and their proven long-term reliability. We have a significant stand-by pool of donors who have been serologically screened each month. As a result, we can organise leukopak production at relatively short notice to mesh with your research schedules.
The cell count we use for our leukopaks is based on the total mononuclear cell count (MNC) contained in the leukopak, rather than the total nucleated cell (TNC) count used by some other leukopak providers. TNC includes neutrophils which typically make up 20-60 % of TNC count and are not desired by most researchers. Therefore, our leukopaks contain an extremely high number of MNC cell populations such as T cells, B cells, NK cells and monocytes.
Our donations are often processed in as little as four hours from collection to optimise cell viability and function.
I have specific donor profiles in mind for my work which could benefit from leukopaks, can research donors help me with this?
Yes, we have a large and diverse donor pool so you can manage donor characteristics for leukopaks which include:
- HLA type and blood group
- Previous infection history with viruses (including CMV and EBV)
- Age (18-66), BMI, Ethnicity, Sex
- Lifestyle factors including diet, nicotine and caffeine usage
It is widely known that donor variability is an important factor in cell therapy manufacturing processes. To help researchers in this and other areas it can be extremely helpful to be able to selectively recall specific donors from whom a leukopak can be collected.
We maintain a significant inventory of frozen leukopaks (and smaller aliquots from these) which are useful as a tool to screen a group of donors to test for application specific suitability. We are then able to recall many of these donors for fresh leukopak collections at a time to suit.
What happens if I need something not listed in your inventory?
At Research Donors, we pride ourselves on flexibility and providing exactly what you need, fast. Should you require anything outside our inventory, we would be happy to discuss any custom options suitable for your research and budget.
Ethics is a very big deal for us. We need to show we are ethically compliant along our drug discovery journey including our use of biospecimens. Can you help?
We have you covered. Our service has been reviewed and approved by an NHS HRA Ethics Committee in the UK. Research Donors is licensed to collect and store blood biospecimens by the Human Tissue Authority.
Our clear consent form is HRA approved and covers genetic research, animal research and IP ownership and more, including commercial use for biomedical research applications.
This all sounds perfect for my next project; how can I order?
You can search our on-line inventory for fresh or frozen leukopaks or request a quote if you need something more bespoke. We will be happy to help make your next project a success!
If you have questions about leukopaks or any aspect of our service, you can use our contact form to get in touch.
Written by Matt Kettle, Commercial Director – Research Donors